
Heavy atom isotope effects involve the substitution of carbon, oxygen, nitrogen, sulfur, and bromine, with effects that are much smaller and are usually between 1.02 and 1.10. Large effects are seen because the percentage mass change between hydrogen and deuterium is great. Normal KIEs for the deuterium effect are around 1 to 7 or 8. This is known as a deuterium effect and is expressed by the ratio k H/k D (as explained above). A very common isotope substitution is when hydrogen is replaced by deuterium. In a KIE experiment an atom is replaced by its isotope and the change in rate of the reaction is observed. Kinetic Isotope Effects (KIEs) are used to determine reaction mechanisms by determining rate limiting steps and transition states and are commonly measured using NMR to detect isotope location or GC/MS to detect mass changes. The increase in energy needed to break the bond results in a slower reaction rate and the observed isotope effect. The resulting molecule has less of a tendency to dissociate.

In summary, the greater the mass the more energy is needed to break bonds. There are two main types of isotopes: stable and unstable (radioactive). The same general procedure can be followed for any isotope substitution. It also describes how these concepts apply to the work that the Department of Energy’s Office of Science conducts as it helps the United States excel in research across the scientific spectrum.\]

DOE Explains offers straightforward explanations of key words and concepts in fundamental science.
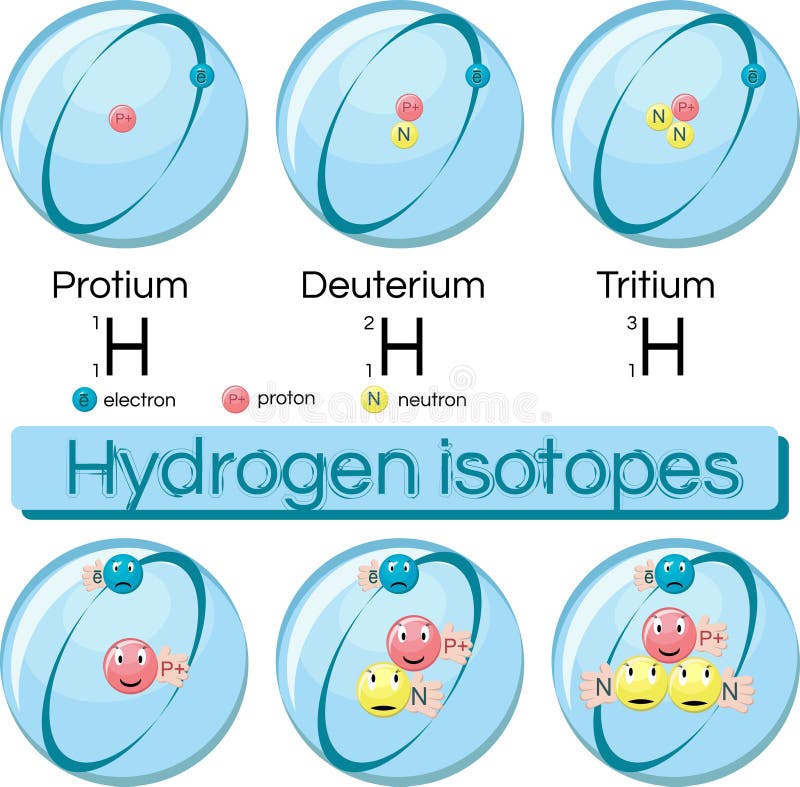
The recently launched Facility for Rare Isotope Beams has completed its groundbreaking first experimental results. National Isotope Development Center ( Isotope Basics). DOE Isotope Development & Production for Research and Applications. The Journey of Actinium-225: How Scientists Discovered a New Way to Produce a Rare Medical Radioisotope. NSAC: Compelling Research Opportunities Using Isotopes. NSAC Report: Meeting Isotope Needs and Capturing Opportunities for the Future. Hydrogen is the only element whose isotopes have unique names: deuterium for hydrogen with one neutron and tritium for hydrogen with two neutrons. Some elements can only exist in an unstable form (for example, uranium). All artificial (lab-made) isotopes are unstable and therefore radioactive scientists call them radioisotopes. There are two main types of isotopes: stable and unstable (radioactive). Finally, it conducts research and development on new and improved isotope production and processing techniques. The program also maintains the infrastructure required to produce and supply priority isotope products and related services. The program produces and distributes radioactive and stable isotopes that are in short supply, including byproducts, surplus materials, and related isotope services. The DOE Isotope Program addresses this need. However, isotopes are not always available in sufficient quantities or at reasonable prices. Isotopes are needed for research, commerce, medical diagnostics and treatment, and national security. They are important in nuclear medicine, oil and gas exploration, basic research, and national security. Isotopes have unique properties, and these properties make them useful in diagnostics and treatment applications. This decay means the amount of carbon-14 in an object serves as a clock, showing the object’s age in a process called “carbon dating.” Carbon-14 is unstable and undergoes radioactive decay with a half-life of about 5,730 years (meaning that half of the material will be gone after 5,730 years). Carbon-12 is stable, meaning it never undergoes radioactive decay. The addition of even one neutron can dramatically change an isotope’s properties. Every element has its own number of isotopes. Carbon occurs naturally in three isotopes: carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons. For example, carbon has six protons and is atomic number 6. The number of protons in a nucleus determines the element’s atomic number on the Periodic Table. Isotopes are members of a family of an element that all have the same number of protons but different numbers of neutrons. Elements have families as well, known as isotopes. A family of people often consists of related but not identical individuals.







 0 kommentar(er)
0 kommentar(er)
